What does a scientist look like?
And Love Island and Radical Chemistry!
Aunt Andi
When asked in 5th grade what I wanted to be when I grew up, I said a science researcher. And if you asked me what that looked like, I’d draw a woman, more specifically, my Aunt Andi.
Aunt Andi was my earliest science influence. I would eagerly await visits with Aunt Andi, which guaranteed the eruption of a baking soda and vinegar volcano.
She finished her PhD in chemistry 50 years ago, at a time when women in science were few and far between.
She held several commercial research and executive R&D positions throughout her career, at Shell, Sherwin Williams, and pharmaceutical companies. She was highly valued and often scouted and scooped, moving around the US to oversee different chemistry departments. Early on in her career, she picked up an MBA by taking classes at night. She knew that science was an investment, and a worthwhile one. She would calculate cost-benefit analyses for different projects to prove their performance. She grinded for a seat at the table. In conference meetings, she would often be drowned out by the men in the room… waiting in quiet frustration for a brilliant point to be made by a male colleague– a point she had made an hour and fifteen minutes prior (it was timed once!)
Aunt Andi is also quick to credit a push for women in science by universities, like MIT (where she went for undergrad), for a part of why she had these opportunities. Yesterday, we spoke about the current scientific political climate. She was emotional, like many of us are. She is a great example of the importance and value of representation in science. Not only did she bring valuable perspectives and knowledge to every room, but she’s also the reason my 5th-grade self pictured a female scientist, and believed I could be one.
Inclusive science benefits everybody and pushes the field forward. There is research to back that diverse teams lead to innovation and productivity.
DEI initiatives help eliminate the gatekeepers to science. DEI cuts won’t just pause progress; the effects will be aggregate and compounding, limiting who feels like they belong and slowing down the pace of progress.
“DEI is not only about diversity training and hiring practices. In the sciences, it is essential and existential to the goal of developing the most robust, talented and highly skilled science workforce in the world.”
Beyond the talent that we will leave behind, underrepresented demographic populations will be understudied. Research is often aligned with the demography of those doing the science. Historically, this means medical research has focused narrowly on a niche population of white men.
Science should be for anyone that wonders ‘why’, asks questions, and oohs and ahs at DIY erupting volcanoes.
Anyways…
One of Aunt Andi’s career accomplishments was working on the team to develop the synthetic polymer in Pull-Ups (Huggies toddler diapers) that makes them elastic. Today’s science lesson will be on polymers, and I will hopefully convince you that radical chemistry is just like Love Island.
Polymers
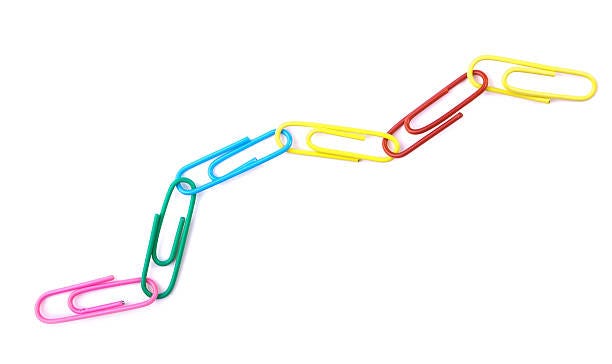
Polymers are long molecules made up of repeating units of smaller molecules called monomers. If the string of paperclips below is a polymer, each individual paper clip is a monomer.
Polymers are all around us. There are natural polymers, like DNA (long chains of nucleotides), and synthetic polymers, like most plastics.
Polymers are so ubiquitous because they are extremely versatile. You can manipulate the number of repeating units (monomers), which monomers you add, or the arrangement to change the properties of your product. Polymers can be soft, hard, rigid, or stretchy.
Polyethylene and some basic Organic Chemistry
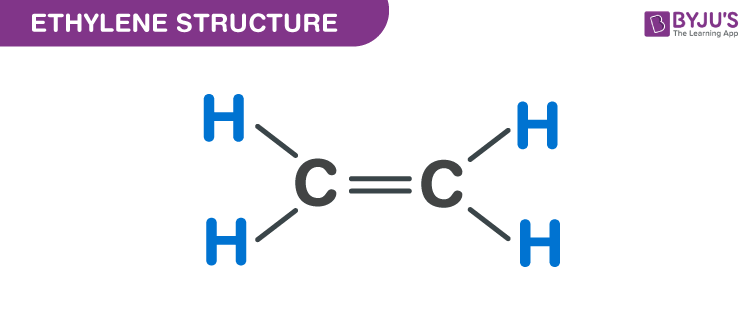
Polyethylene is the simplest synthetic polymer and one of the most common plastics worldwide. It is made by linking small units of ethylene together. Ethylene is a monomer that forms the polymer, polyethylene. Ethylene is two carbon atoms double-bonded together. Each carbon atom is also bonded to two hydrogen atoms. In chemistry diagrams, bonds are represented with lines. Each bond (these are covalent bonds), or line, represents 2 electrons. These 2 electrons are being shared between the atoms on either end of the line. Covalent bonds form in order to satisfy the energy/electron requirements of each atom.
Imagine that when each carbon is alone, not in a bond, they are not entirely satisfied. They spend their time looking for a partner (or two or three) so they can all mutually satisfy each other.
For the sake of the Love Island analogy, I will equate a chemical bond to a connection in the Villa, and I will give each of our carbon atoms a Love Island player comp. We will pretty much ignore the hydrogens. Carbons are the main characters of Organic Chemistry.
Okay, so before we go into step 1 of this reaction, let’s look at what the final product (the chain of paperclips) will look like. This is where Love Island diverges slightly from polymerization. Instead of single, vulnerable islanders being kicked off, imagine that they just go on forming new connections until you have a long chain of connecting love triangles. (That could actually be a fun show too: Polygamy Island).
With that in mind, this is what our end product looks like:
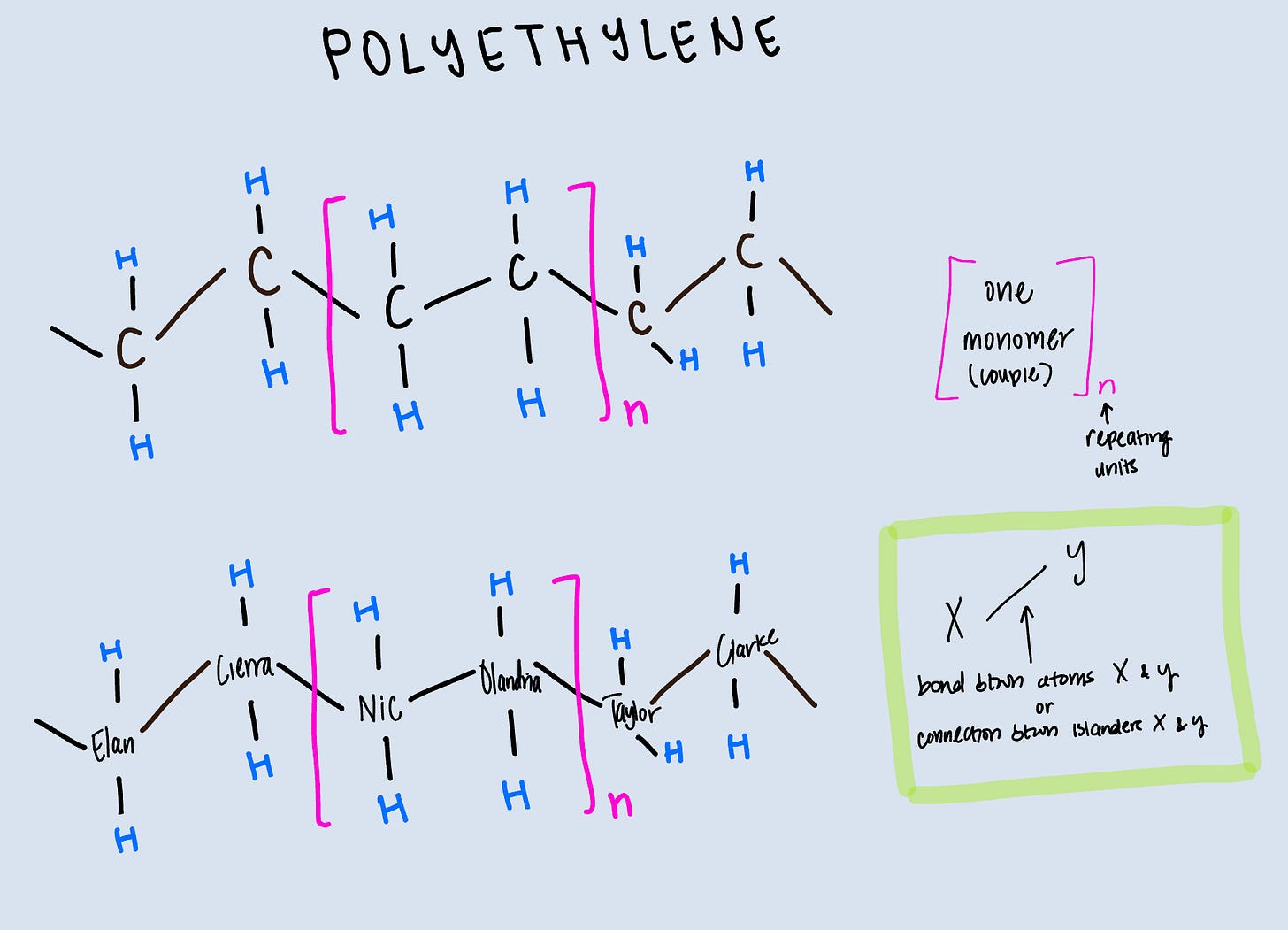
I have shown what one monomer looks like, in the pink box. That monomer will repeat n (any number) times. Again, the lines are bonds/connections. So you can see we have a long chain of carbons (islanders) all sharing electrons via covalent bonds (exploring connections with each other).
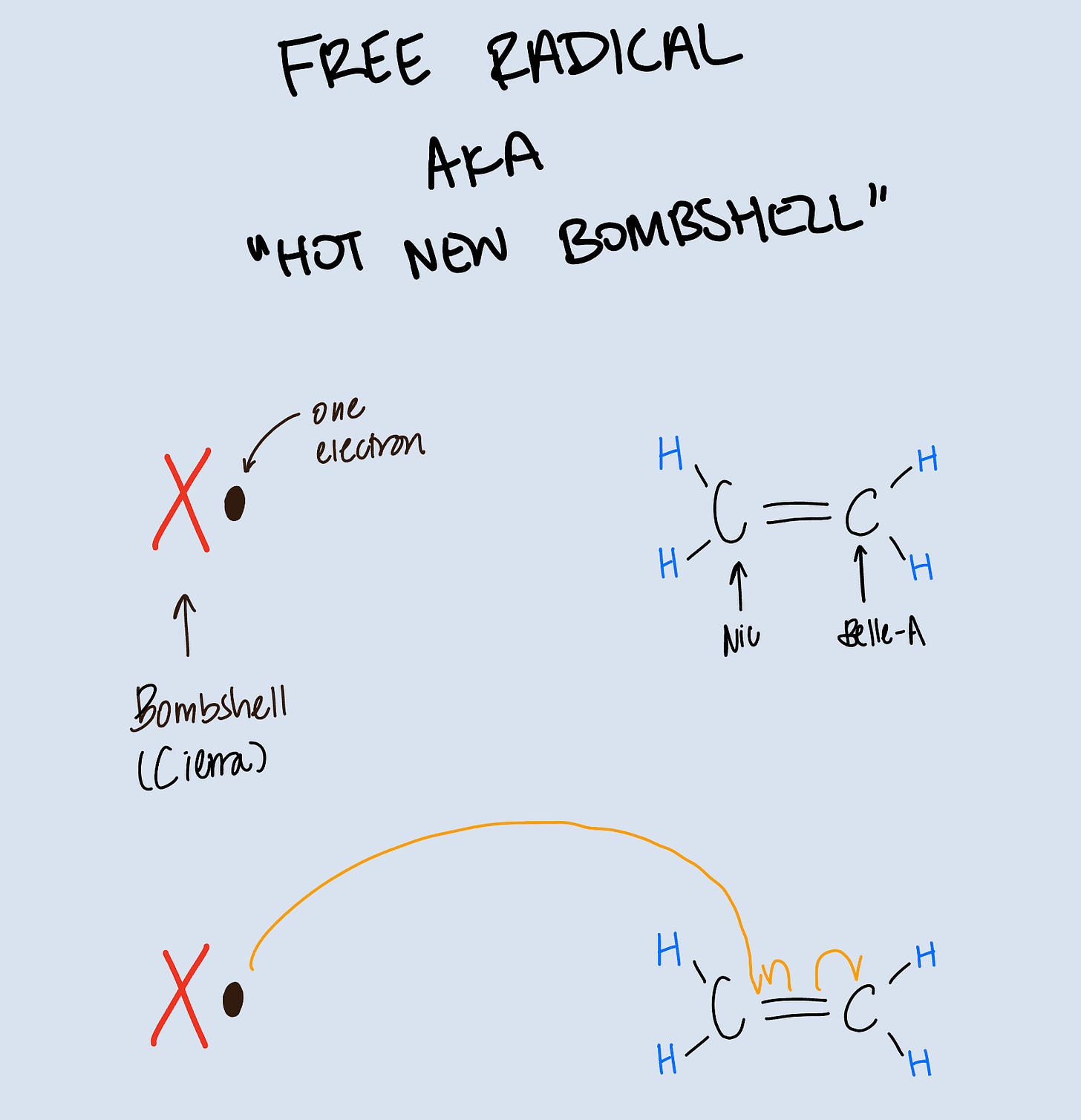
A hot new bombshell has entered the villa…
Now let’s see how we reach that final product. First, we introduce a free radical. Free radicals are atoms carrying one unpaired electron. They are super unstable and therefore highly reactive. They will steal electrons and break bonds to reach a more stable configuration. They are literally bombshells: desperate, single, vulnerable, willing to home-wreck.
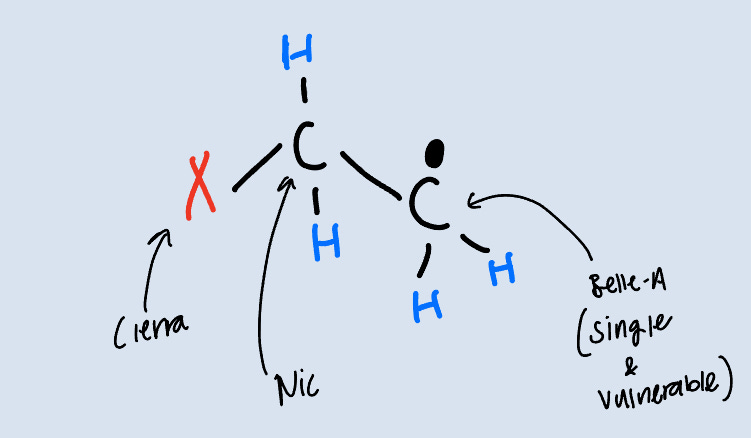
Once again, we have our Ethylene molecule. If we introduce a free radical to this reaction, it will break the carbon-carbon double bond and form a new bond with one of the carbons, forcing a single electron onto the other carbon and making that second carbon a radical.
Think episode 2, Cierra arrives in the Villa. Even though Nic is coupled up with Belle-A, Cierra makes a move on him and he can’t resist. He couples up with Cierra and is now in a love triangle. Belle-A is feeling vulnerable because her man just got stolen and so she starts looking around to see if she can do the same thing to someone else.
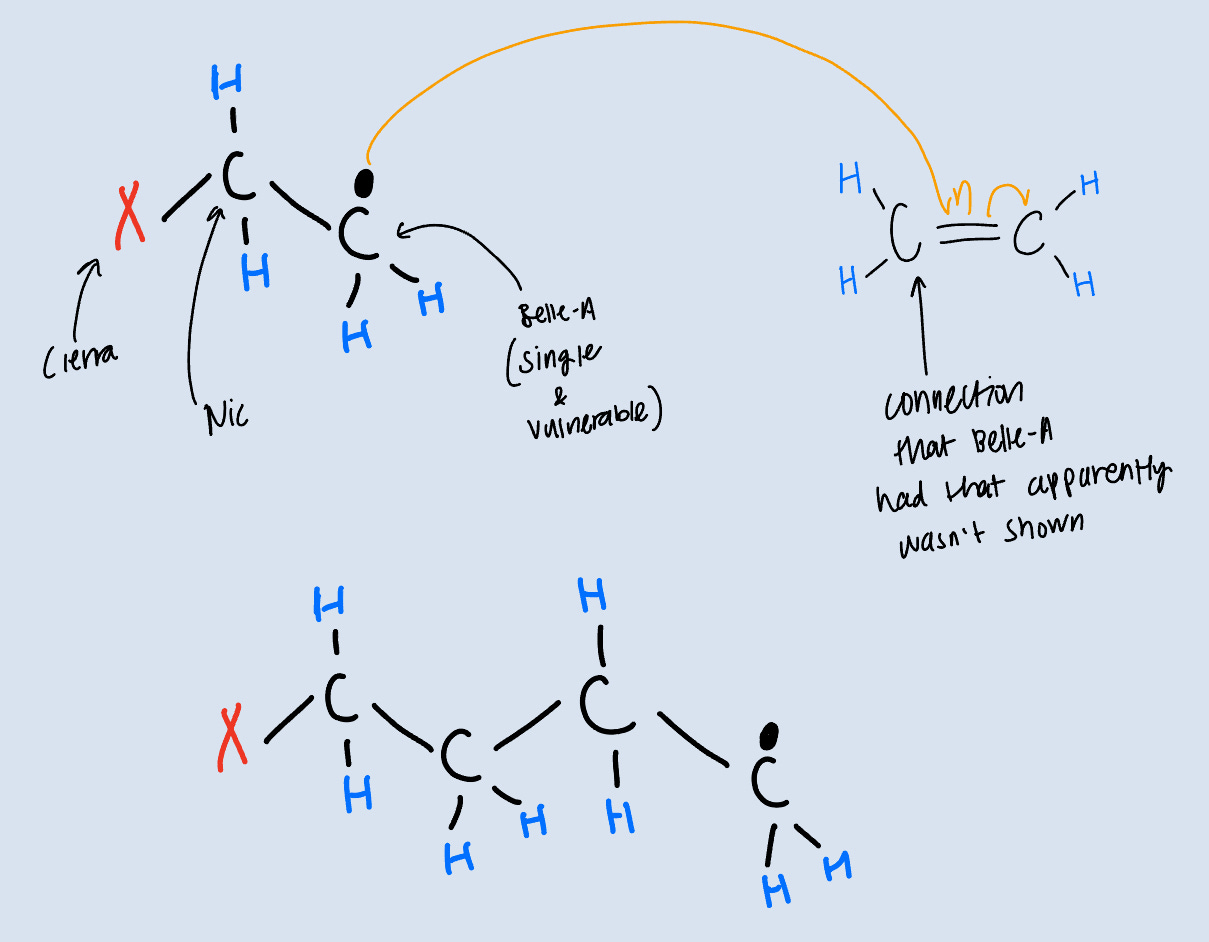
Belle-A makes a move on another person (the connection she had that apparently wasn’t aired…) and this process occurs again and again until an organic chemist in a lab somewhere terminates the reaction.
Hopefully you can see how we would arrive at our product:
I hope that made sense, or at the very least didn’t rot your brain. I can’t believe I just wrote this. There is now a Love Island note in my Orgo 2 folder on my iPad. Perfect.
Peace out prank:
I read about this funny chemistry prank in the book The Disappearing Spoon. Gallium is a metal with a super low melting point (85.6 °F). Scientists would have people over for tea and give them a Gallium spoon which would be solid at room temp, but as they went to stir their tea, it would melt! LOL get wrecked.


I'm not sure why my edit won't process but I mean to say that radicals have one unpaired electron. Thx ;-)